Hydrotalcite-like compounds (HTLC), also known as layered double hydroxides, contain water and anions in the nanoscale interlayers. They are widely used as catalysts, adsorbing agents, electrode modifiers, and so on. These usages are correlated with physical-chemical processes in interlayers (e.g., anion exchange, protonic conduction, hydration, and dehydration), which are controlled by structure and dynamics of nanoconfined water molecules. Ab initio and Raman spectroscopy studies showed the structure of intercalated water is similar to that of ice Ih. As we know, the difference between diffusion ways of atoms in liquid and solid lattice is that atoms exhibit continuous motions in liquid while they jump between lattice sites in solid. Since water in LDH shares similarity with ice, does it diffuse like atoms in solid? It is an interesting scientific question.
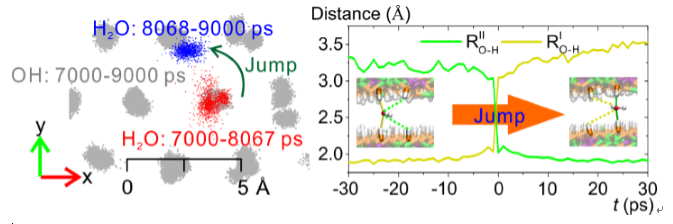
Through molecular dynamics simulation studies, Dr. Meng Chen of Guangzhou Institute of Geochemistry found water intercalated in HTLC (Mg2Al(OH)6Cl?mH2O) diffuses in a jumping way as atoms in solid lattice. Intercalated water exhibits a monolayer structure. A water molecule is mostly fixed in a hydroxyl group site, as an acceptor of hydrogen bonds donated by the upper and lower hydroxyl groups simultaneously, and as a donator donating hydrogen bonds to adjacent Cl- ions or water molecules. Thus, it exhibits a tetrahedral coordination structure, similar to ice. So the water molecule vibrates with a similar frequency as atoms from hydroxyl groups. However, due to occasional hydrogen bond exchanges, it loses hydrogen bonds from the two hydroxyl groups and accepts hydrogen bonds from another two groups in an adjacent site. Thus, the water molecule jumps from one hydroxyl group site to another. A jump is rapid bur rare. On average it takes ~104 ps for a jump to happen on a water molecule. The diffusion coefficient derived by the jump model is of the same order (~10?9 cm2/s) as that obtained by fitting the mean-square displacement, revealing water diffusion in the confined monolayer is largely contributed by a series of jump events.
This paper has been published in The Journal of Physical Chemistry C. This work was financially supported by the National Natural Science Foundation of China, etc.
Chen, M., Shen, W., Lu, X.C., Zhu, R., He, H.P., and Zhu, J.X. (2016) Jumping Diffusion of Water Intercalated in Layered Double Hydroxides. The Journal of Physical Chemistry C, 120(23), 12924-12931.