| Research Hightlights |
| Events |
| Focus News |
| Upcoming Events |
| Location: Home > News > Research Hightlights |
| Identification of some new oxygenated precursors of botryococcanes in oilshale samples in the Maoming Basin | TEXT SIZE: A A A |
Botryococcanes are diagnostic biomarkers for race B of hydrocarbon-riched microalgae—Botryococcus braunni, in which its molecular geochemical studies are of important to illustrate the key information on the paleoecologic reconstruction, evaluation of oilshale and shale oil resource, as well as the development of future biofuels.
In 2018, according to the team works of Dr. Liao Jing and Prof. Lu Hong in Guangzhou Institute of Geochemistry, Chinese Academy of Sciences (GIG-CAS), four novel botryococcanoids with unique skeleton containing a methyl group positioned β- to the sole quaternary carbon had been identified in the samples oil shale in the Maoming basin, SW China, based on the evidentially structural determination of NMR data. Consequently, an unusual biosynthetic pathway with cyclobutanation through a cyclobutane ring intermediate, rather than a conventional cyclopropane ring intermediate, was proposed for the formation of these botryococcanoids.
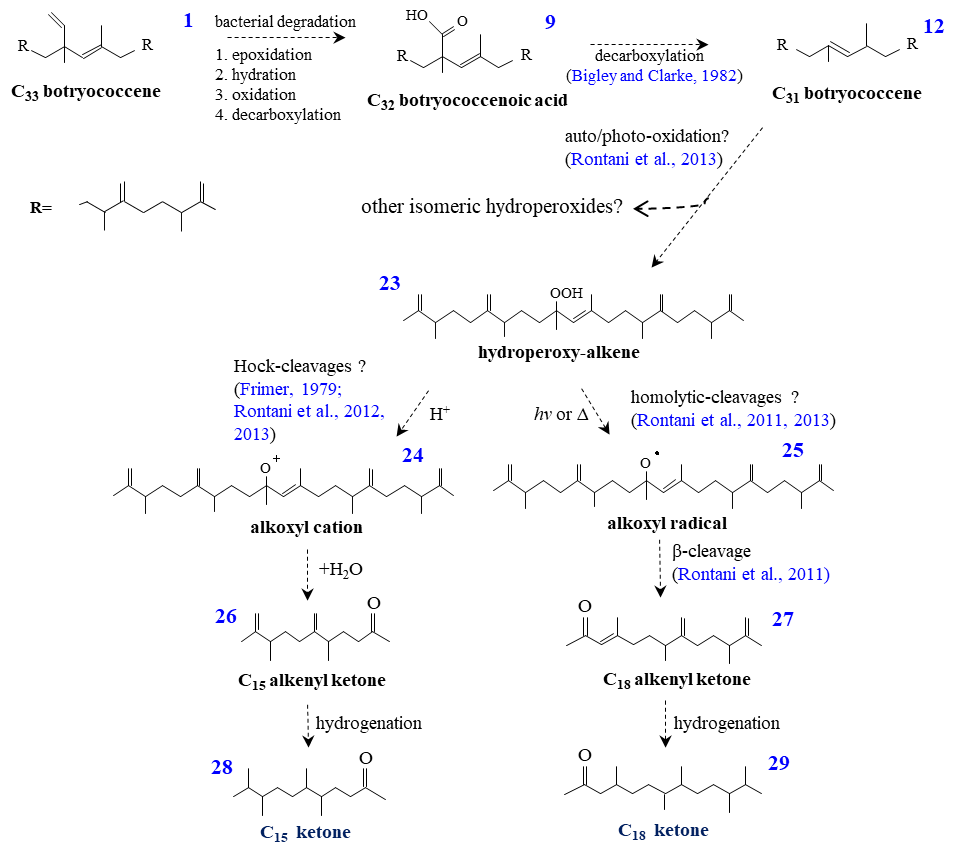
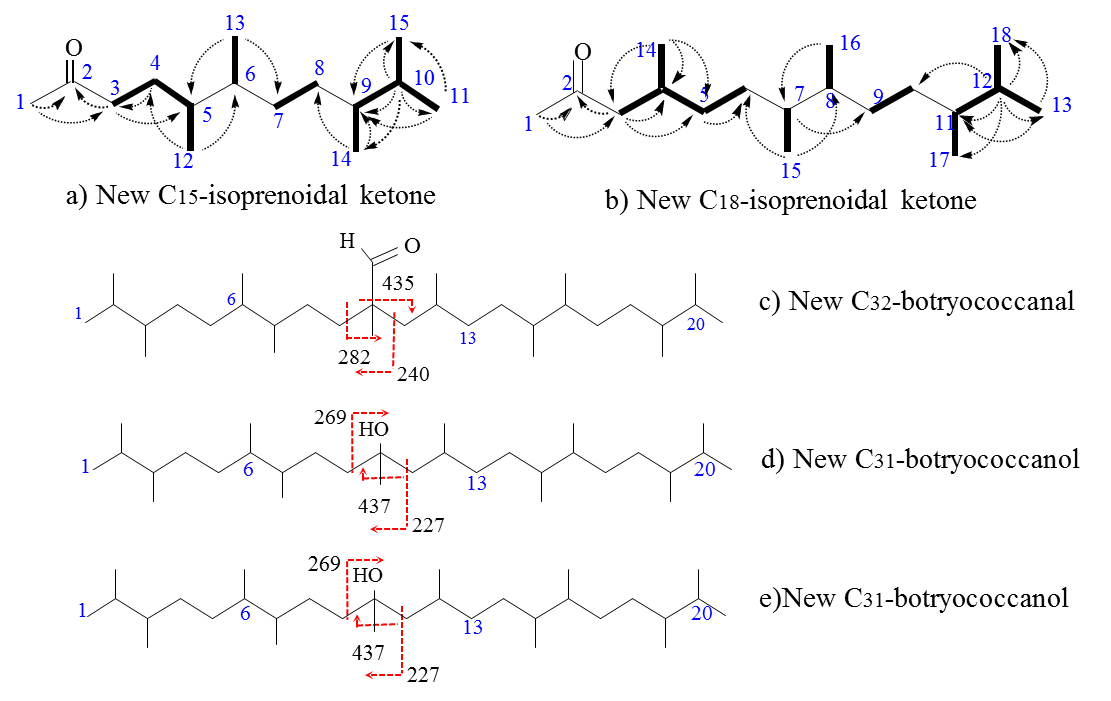
In 2020, five new oxygenated intermediates of these novel botryococcanes have been further identified and reported by the team. Based on the solid structural identification and conformation of these oxygenated intermediates, a more explicit post-diagenetic pathway can be also built for the formation of these novel botryococcanes discovered in Maoming, which was illustrated in detail as below: 1) Identifications of the new C15-, C18-isoprenoidal ketones and three new oxygenated intermediates: the structures of the new identified C15-, C18-ketones named as 5,6,9,10-tetramethyl-undecyl-2-ketone and 4,7,8,11,12-pentamethyl-tridecyl-2-ketone, respectively (Fig. 1a, b), were determined by NMR spectra; The structures of the three oxygenated intermediates of C32-botryococcanal (Fig. 1c) and two C31-botryococcanols (Fig. 1d, e), were identified based on the fragmental deduction of their mass spectra.
(Image by LIAO jing) Fig. 1 Skeletons of the new C15、C18-isoprenoidal ketones and three new oxygenated derivatives 2) Based on the structural consolidation of the new identified oxygenated botryococcanoids, a post-biological/sedimentary diagenetic pathway can be reasonably proposed for the formation of the novel botryococcanes early reported. ①Novel C33-botryococcene (2) is formed due to the ② In the above mentioned diagenetic pathway, as the final intermediate product, the internal double bond of the C31-botryococcene (12) had easily and preferentially undergone the abiotic degradation (photo-oxidation and autoxidation), as a result, firstly produce hydroperoxide (23) and subsequent C15- and C18-alkenyl ketones (26,27) due to Hock cleavages and Homolytic cleavages (Fig. 3). Finally, the identified C15-, C18-ketones (28,29) can be available by diagenetic hydrogenation.
(Image by LIAO jing) Fig. 2 Post-biological/sedimentary diagenetic pathways for the formation of the novel C33-, C32-, C31-botryococcanes.
(Image by LIAO jing) Fig. 3 Pathways for the formation of the novel C15-, C18-ketones. These results have been published in two papers on “Organic Geochemistry” after peer reviews, and entitled as “Two new isoprenoidal ketones related to Botryococcus braunii in the Chinese Maoming Basin” (https://doi.org/10.1016/j.orggeochem.2019.103946) and “Identification of a novel undecamethylhenicosane and three oxygenated precursors in a Maoming Basin shale” (https://doi.org/10.1016/j.orggeochem.2020.103974), respectively. |
||
|
|
||
 condensation (cyclobutanation) during the incorporation of two FPP (farnesyl diphosphates, 1). Bacterial degradation of the terminal double bond connected to the sole quaternary carbon in C33 botryococcene can produce oxygenated derivatives of C33-botryococcene (3,4,5,6,7) (Fig. 2). Subsequent and continuous degradation around the sole quaternary carbon account for the formation of oxygenated derivatives of C32-botryococcene (8,9), as well as the formation of C31-botryococcene (12) and its oxygenated derivatives (15,16). Through the diagenetic hydrogenation, these oxygenated derivatives can further produce the identified C33-botryococcanone (10), C32-botryococcanal (11) and two C31-botryococcanols (19,20) (Fig. 2). In the end, ongoing hydrogenation and dehydration contribute to the formation of the C31-33 botryococcanes (17,18,21,22) (Fig. 2).
condensation (cyclobutanation) during the incorporation of two FPP (farnesyl diphosphates, 1). Bacterial degradation of the terminal double bond connected to the sole quaternary carbon in C33 botryococcene can produce oxygenated derivatives of C33-botryococcene (3,4,5,6,7) (Fig. 2). Subsequent and continuous degradation around the sole quaternary carbon account for the formation of oxygenated derivatives of C32-botryococcene (8,9), as well as the formation of C31-botryococcene (12) and its oxygenated derivatives (15,16). Through the diagenetic hydrogenation, these oxygenated derivatives can further produce the identified C33-botryococcanone (10), C32-botryococcanal (11) and two C31-botryococcanols (19,20) (Fig. 2). In the end, ongoing hydrogenation and dehydration contribute to the formation of the C31-33 botryococcanes (17,18,21,22) (Fig. 2).