Organosulfates (OSs), which could contribute to the total mass of ambient organic aerosols by as much as 30 %, are important components of the atmospheric secondary organic aerosols, significantly affecting atmospheric environment and climate. However, our understanding of the hygroscopicity and cloud condensation nuclei activity of OSs aerosols are still rather limited.
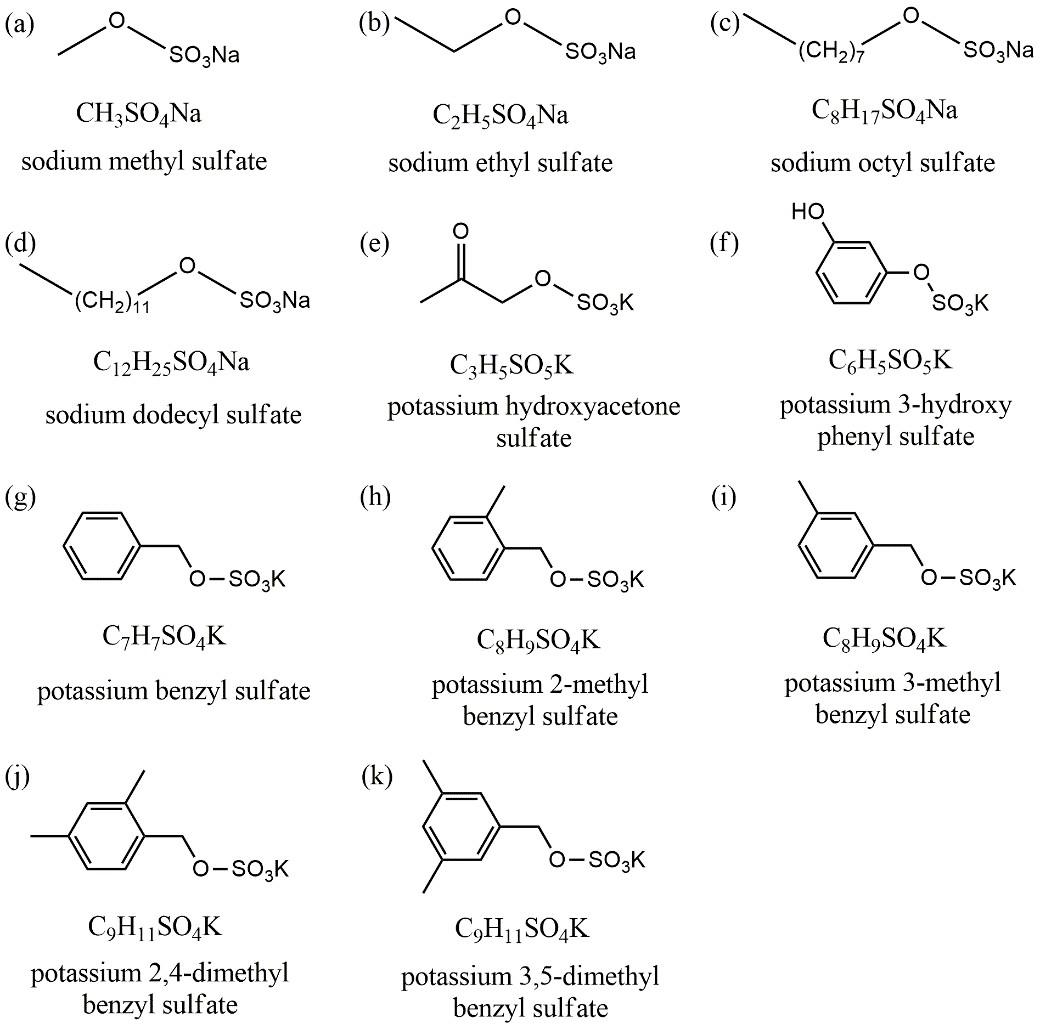
Figure 1. Chemical formulas and molecular structures of organosulfates investigated in this study.
To explore this issue, Chao Peng and Mingjin Tang from the State Key Laboratory of Organic Geochemistry, employed three complementary techniques, including a vapor sorption analyzer (VSA), a humidity tandem differential mobility analyzer (H-TDMA) and a cloud condensation nuclei counter (CCNc), to characterize interactions of 11 OSs (Figure 1) with water vapor under sub- and super-saturated conditions. They found large variations in the hygroscopicity of different OSs species. To be specific, sodium methyl sulfate (methyl-OS), sodium ethyl sulfate (ethyl-OS), sodium octyl sulfate (octyl-OS) and potassium hydroxyacetone sulfate are able to absorb water vapor with increasing relative humidity (RH), no significant water uptake was observed in sodium dodecyl sulfate (dodecyl-OS) and other six potassium aromatic organosulfates even when RH was up to 90%. For methyl-, ethyl- and octyl-OS, the hygroscopicity and CCN activity decreased with alkyl chain length, suggesting that the addition of hydrophobic hydrocarbon functional groups to OS would significantly reduce their hygroscopicity.
Based on the hygroscopic growth factors derived from H-TDMA measurements and CCN activities derived from CCN measurements, they calculated the single hygroscopicity parameter, κ, of methyl-, ethyl- and octyl-OS aerosols under sub- (κgf) and supersaturated (κccn) conditions, and the results were summarized in Figure 2. For methyl- and ethyl-OS aerosols, κccn values agree well with those derived from H-TDMA measurements (κgf) with relative differences being <25 %, whereas κccn was found to be ~2.4 times larger than κgf for octyl-OS, and they show that the solubility limit and surface tension reduction may both contribute to such a discrepancy as observed.
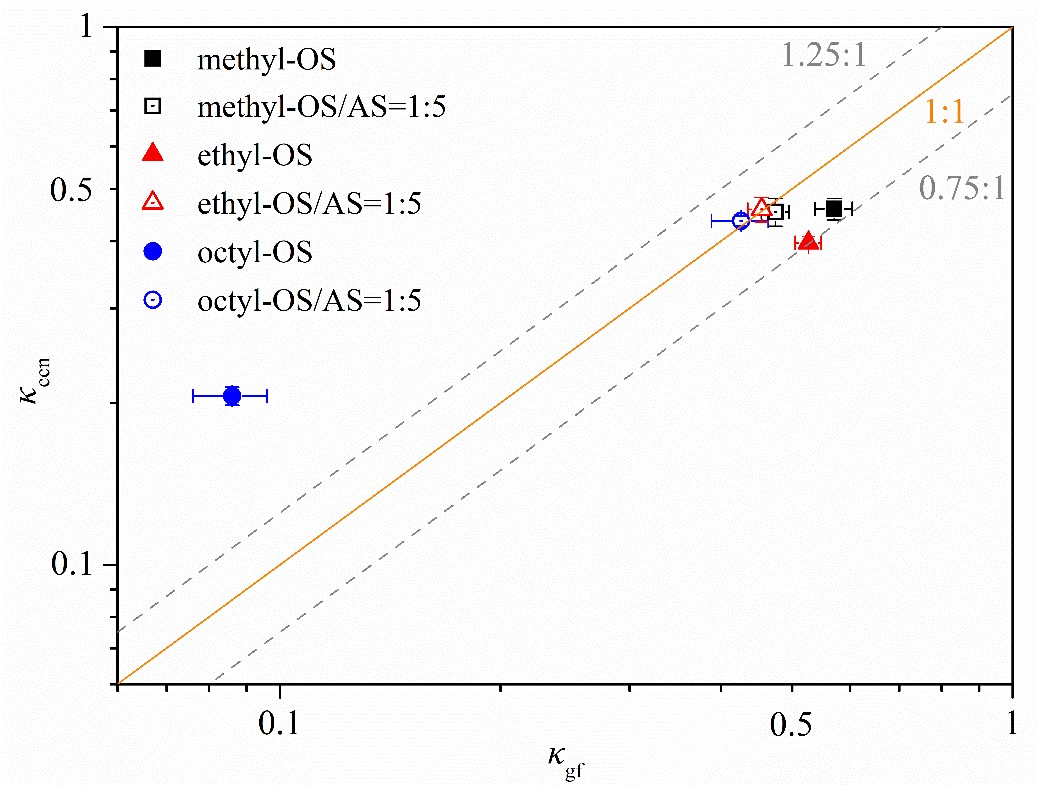
Figure 2. Comparison of κ values derived from hygroscopic growth (κgf) with those derived from CCN activities (κccn) for methyl-, ethyl- and octyl-OS aerosols as well as their internal mixtures with ammonium sulfate (the mass ratio was 1:5).
Reference:Peng, C., Razafindrambinina, P. N., Malek, K. A., Chen, L. X. D., Wang, W. G., Huang, R. J., Zhang, Y. Q., Ding, X., Ge, M. F., Wang, X. M., Asa-Awuku, A. A., and Tang, M. J.: Interactions of organosulfates with water vapor under sub- and supersaturated conditions, Atmos. Chem. Phys., 21, 7135-7148, 2021.
https://acp.copernicus.org/articles/21/7135/2021/

