Clouds and aerosols are interconnected while affecting the surface temperature of our planet by reflecting the sunlight (cooling effect) and trapping the heat (warming effect). The cloud droplets and aerosol particles contain a large fraction of organic compounds which are transformed by chemical reactions and affect their composition and their physical and chemical properties which, in turn, influence the climate. Liquid water content (LWC) of clouds which is expressed as water per volume of air (g m-3) can be considered as ideal solution because of low ionic strength. Indeed, ionic strength in remote cloud droplets varies between 7.5 x 10-5 and 7.5 x 10-4 mol L-1, and in polluted cloud droplets it is somewhat higher ranging from 5 x 10-3 to 1 x 10-2 mol L-1. In marine aerosols the ionic strength can reach up to 6 mol L-1 and in the aerosol particles of urban environment it is substantially higher, 18.6 mol L-1. During severe Beijing Haze with PM2.5 levels higher than 300 μg m-3 the ionic strength in the wet particles varies between 13 and 43 M. This high ionic strength can affect the kinetics and products distribution within the aerosol deliquescent particles and, thus, it can affect aerosol composition and optical properties.
To better predict and explain the haze formation processes, studies focused on ionic strength effects on aqueous phase reactions, photolytic degradation of organic compounds, and gas-liquid heterogeneous processes, are urgently needed.
In two recent papers published in Environmental Science and Technology by the MPhil students Yiqun Wang and Huifan Deng, it has been shown that ionic strength can affect the heterogeneous kinetics of atmospheric oxidants such as ozone (O3) and nitrogen dioxide (NO2) with layers of methoxyphenols and fatty acids, respectively, and favors the formation of “brown carbon” compounds.
Ultrahigh-resolution electrospray ionization FT-ICR MS indicates the formation of oxygenated aliphatic CHO compounds and multicore aromatics upon heterogeneous ozone processing of ortho-vanillin (o-VL) in the dilute aqueous phase. The addition of SO42- that is typical for the liquid core of aerosol deliquescent particles increases the formation of condensed aromatics, but also organosulfates (-OSO3H) arose upon ozone reactions with o-VL. The addition of NO3- ions substantially favors the formation of multicore aromatic compounds through heterogeneous ozone processing of o-VL. More importantly, the addition of NO3- ions favors the formation of organonitrates, which are potential components of the light-absorbing organic matter (“brown carbon”) that is still poorly characterized and may have the potential to affect both climate and air quality (Wang et al., 2021).
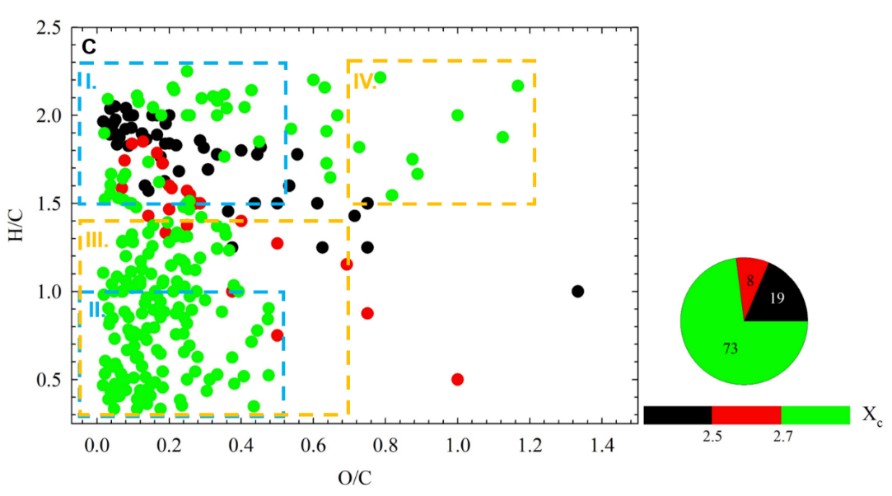
Figure 1: The van Krevelen graph for the CHO, CHON, and CHOS groups of compounds, formed upon dark heterogeneous reaction of o-VL in presence of NO3- (Ieff = 0.32M). The color-coding indicates the calculated DBE values. The aromaticity equivalent (black with Xc < 2.5, red with 2.5 ≤ Xc < 2.7, and green with Xc ≥ 2.7) follows the color bar, while the pie chart shows the percentage value of each color-coded group in the sample. See Wang et al., 2021 for the description of regions I to IV.
Fatty acids are ubiquitous constituents of grime on urban and indoor surfaces, and they represent important surfactants on organic aerosol particles in the atmosphere. Deng et al. (2021) assessed the heterogeneous processing of NO2 on films consisting of pure oleic acid (OA), or a mixture of OA and representative salts for urban grime and aerosol particles, namely Na2SO4 and NaNO3. The uptake process of NO2 on OA gives HONO as reaction product, and the highest HONO production was observed upon heterogeneous reaction of NO2 with OA in the presence of nitrate (NO3-) ions. The formation of gaseous nitroaromatic compounds was also enhanced in the presence of NO3- ions upon light-induced heterogeneous processing of NO2 with OA as revealed by membrane inlet single-photon ionization time-of-flight mass spectrometry (MI-SPI-TOFMS). Considering that fatty acids are ubiquitous surfactants in the atmospheric particles and on urban and indoor grime, the obtained results suggest that fatty acids can contribute to urban- and indoor air pollution through heterogeneous conversion of NO2 into N-containing organic compounds including nitrous acid (HONO) and brown carbon compounds.
The detailed knowledge of HONO formation processes is paramount with respect to the urban and indoor air chemistry because HONO is the main source of OH radicals through its photolysis in the urban environment and in sunlit areas of the indoor environment. Especially, the formation of N-containing organic compounds by the reaction of NO2 with the OA in the presence of nitrate ions can exhibit health problems. The presence of NO3- ions during the heterogeneous NO2 processing of OA stimulates the formation of nitroaromatic compounds, which are potential light-absorbing (brown carbon) compounds that could affect the climate through positive radiative forcing (warming).
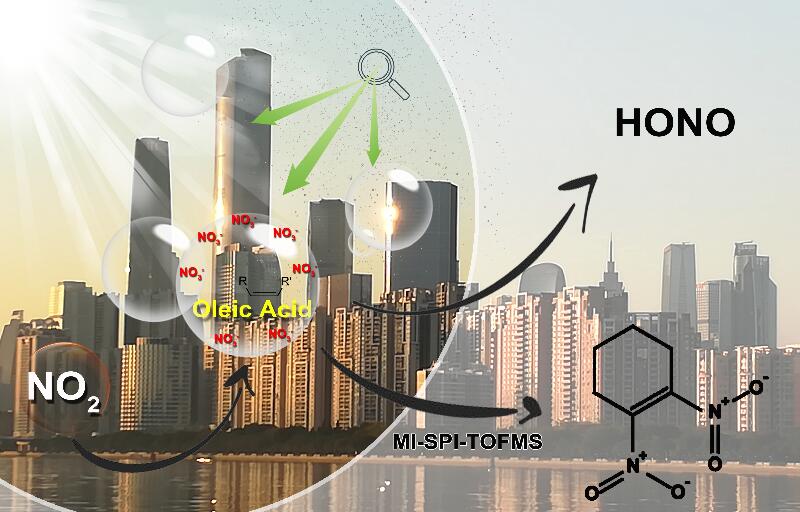
Figure 2: Simplified illustration of the influence of nitrate ions on heterogonous processing of NO2 with the oleic acid and consequent formation of HONO and brown carbon compounds.
The research team of Sasho Gligorovski built recently in the State Key Laboratory of Organic Geochemistry (SKLOG), Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, focuses on atmospheric heterogeneous processes and recent research progresses have been published in the journals of Environmental Science and Technology and Atmospheric Environment. These researches are granted by NSFC and SKLOG.
Further reading:
Y. Wang, M. Mekic, P. Li, H. Deng, S. Liu, B. Jiang, B. Jin, Davide Vione, S. Gligorovski, Ionic Strength Effect Triggers the Brown Carbon Formation through Heterogeneous Ozone Processing of o-Vanillin, Environ. Sci. Technol., 2021, 55, 8, 4553–4564.
H. Deng, J. Liu, Y. Wang, W. Song, X. Wang, X. Li, D. Vione, S. Gligorovski, The Effect of Inorganic Salts on N-Containing Organic Compounds Formed by Heterogeneous Reaction of NO2 with Oleic Acid, Environ. Sci. Technol., 2021, doi.org/10.1021/acs.est.1c01043.
M. Mekic, S. Gligorovski, Ionic strength effects on heterogeneous and multiphase chemistry: Clouds versus aerosol particles, Atmos. Environ., 2021, 244, 117911.
G. Loisel, M. Mekic, S. Liu, Y. Wang, H. Deng, S. Gligorovski, Ionic strength effect on oligomer formation through photochemical degradation of vanillin in liquid water of aerosol particles, Atmos. Environ., 2021, 246, 118140.