| Research Hightlights |
| Events |
| Focus News |
| Upcoming Events |
| Location: Home > News > Research Hightlights |
| Photochemical SO2 conversion on building surfaces contribute to air pollution in megacities | TEXT SIZE: A A A |
Organic sulfur (OS) compounds have been globally identified in ambient secondary organic aerosols (SOA), but their contribution to organic mass is not well quantified, and the current models considerably underestimate ambient SOA formation. Organic sulfur compounds are surface active and may also play a role in cloud formation. To shed new insights into the chemistry of this complex system, Sasho Gligorovski and his scientific team from the State Key Laboratory of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences recently suggested that sunlight-induced heterogeneous SO2 processing on authentic urban grime of glass windows collected in a megacity Guangzhou, China, can be an important additional source of OS compounds. This study ‘first of its kind’ claiming that natural sunlight triggers release of OS compounds from urban grime on building surfaces.
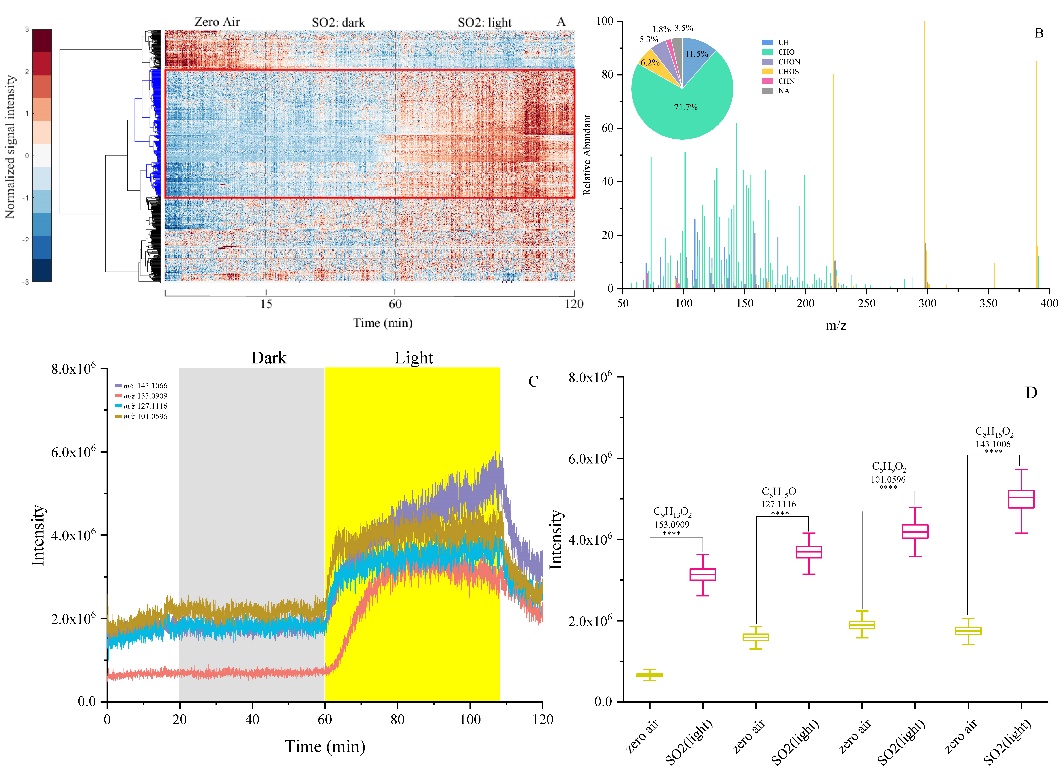
Figure 1: Mass spectrometric data representing the SO2 chemistry with urban grime. The measured reactive uptakes of SO2 on urban grime increase with the relative humidity due to changing diffusion coefficients of SO2 and fatty acids as the urban grime becomes less viscous. The kinetic multi-layer model of aerosol surface suggested that the uptake coefficients are likely to be dependent on a variety of factors such as the relative humidity, the concentrations of a variety of reactive species in the urban grime and the rate of loss and replenishment of these species in urban grime.
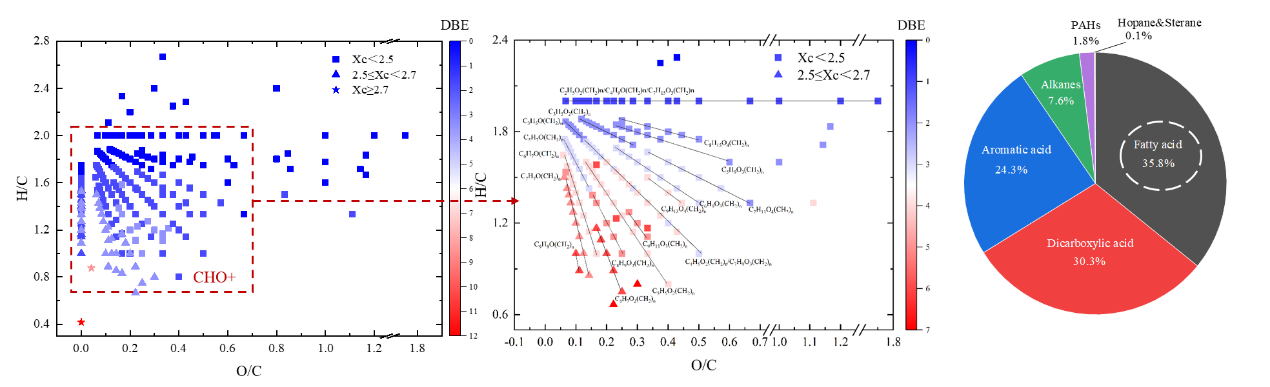
Figure 2: Van Krevelen plot for the observed organic compounds in ESI+ and the composition of urban grime by GC/MS. Real-time measurements of the product compounds released in the gas-phase by the heterogeneous reaction of SO2 with urban grime at 70% RH, in the dark and under UV-A-irradiation were assessed by ultra-high-resolution mass spectrometry in both, negative (ESI–) and positive (ESI+) ionization mode (Figure 1). The gas chromatography mass spectrometric analysis of the organic composition of the urban grime indicated that the formation of the organic sulfur compounds through photochemical SO2 chemistry is initiated by fatty acids that exhibit highest fraction of 35.8 %, followed by dicarboxylic acids (30.8 %), aromatic acids (24.3 %), and alkanes (7.6 %) (Figure 2).
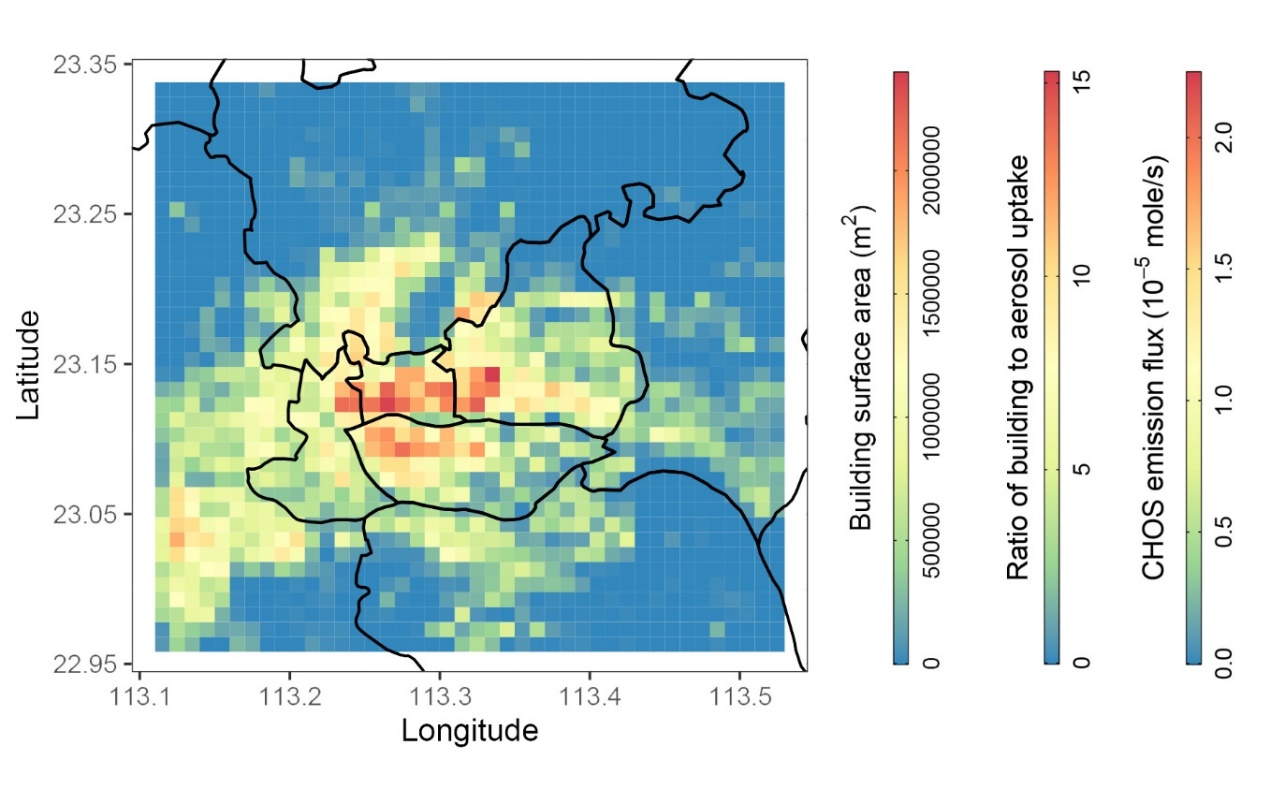
Figure 3: Distribution of building surface areas, ratio between building surface uptake and aerosol surface uptake, and gaseous CHOS emission fluxes from building surfaces in the city of Guangzhou. The surface area of urban buildings in the city of Guangzhou was also estimated based on a building dataset which helped to estimate the fluxes of SO2 by building surfaces. Building uptake fluxes are up to 15 times aerosol uptake fluxes at the core area of Guangzhou. The calculations suggest formation of 20 ng m-3 of OS compounds based on the light-induced heterogeneous reactions of SO2 with building surfaces in Guangzhou (Figure 3). This value is on the same order of magnitude with organosulfur concentrations measured in Chinese megacities, suggesting that photochemical SO2 conversion on ubiquitous building surfaces may be a non-negligible source for OS aerosol concentrations in megacities (Figure 3). The paper was published by Huifan Deng a second year PhD student as a first author on 28 September in Science Advances (https://www.science.org/doi/10.1126/sciadv.abq6830). |
||
|
|
||